Self-Healing Polymers
Dive into the uses, mechanisms, and chemistry behind these cutting-edge materials, and the inevitable challenges that such creations might face in practice
There are few scenarios in which something breaking is a good thing. From throwing your phone at the wall because you didn’t quite manage to win that showdown to fracturing your toe on the sports pitch, cracks tend to be a common source of pain, annoyance, chagrin, even catastrophes. However, chemistry has provided an answer to this tormentor of everyday life – self-healing polymers. In this article, prepare to dive into the uses, mechanisms, and functionality of these life-saving materials, and the inevitable challenges that such creations might face in practice.
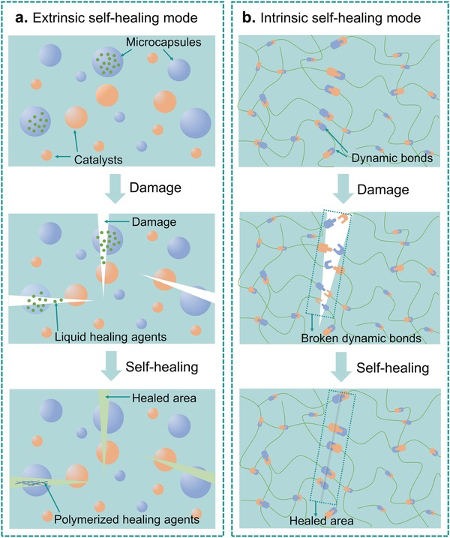
A self-healing polymer is pretty much exactly what it says on the tin – an artificial material that can automatically repair damage to itself without external intervention. Imagine falling and grazing yourself – your body will automatically respond, firstly by stimulating the activity of platelets, then having them travel to the injury site, and finally making them trigger and undergo chemical reactions to form a clot in the wound to prevent blood from escaping. These polymers work in a similar way – the damage triggers a response, which is enacted first by transport of materials to the damaged area, then having them undergo a chemical reaction to repair the damage. The reactions often require specific temperatures, meaning the mechanism is different for each polymer. However, they can roughly be classified as capsule-based, vascular-based, or intrinsic mechanisms.

Capsule-based Healing
The first - capsule-based healing - involves capsules, each containing chemical monomers known as a ‘healing agents,’ being installed throughout the polymer. When molecular-level cracks occur within the material, bonds are broken, causing the capsules to break apart and release the healing agents into the crack plane. The healing agents then react with catalysts embedded in the material and a polymerisation reaction is triggered, forming a polymer from the healing agents that fills up the crack and restores the material’s original functionality.
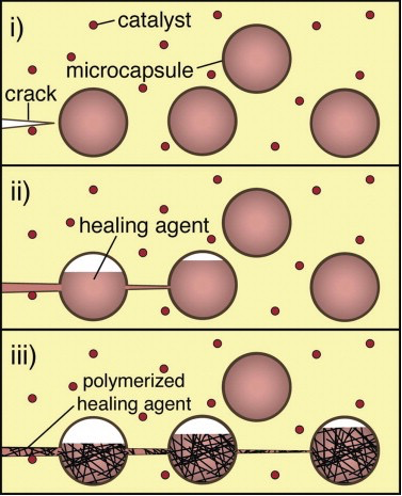
While the catalyst allows the reaction to occur at room temperature, they tend to be expensive, limiting the viability of large-scale use. Furthermore, the healing agent must have a necessary viscosity (i.e. flow fast enough) to fill the entire crack before it is polymerized by the catalyst, but the reaction must occur fast enough that the crack does not expand. Finally, the capsule wall must not be too thick that it does not rupture with the crack, but must be thick enough that it does not rupture prematurely.
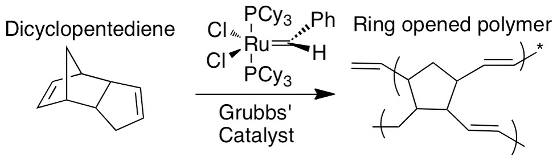
An example of a healing agent used in these polymers is dicyclopentadiene (or DCPD), with the molecular formula C10H12, and its corresponding catalyst is Grubbs Catalyst (C43H72Cl2P2Ru). The DCPD monomers are normally unreactive and are separated from the catalyst by the capsule walls. However, upon being released into the material, they come into contact with the catalyst and form a polymer. The polymerisation reaction can be seen here:
Vascular-based Healing
The second approach – vascular based healing – is similar to the first, involving the storage, release, and polymerisation of monomers at a crack site. However, rather than sitting in capsules, the monomers are continuously flowing through a network of hollow channels, like blood vessels in the human body, made of fibres or glass. These may be single channels, or a branched network. Separate channels hold hardening agents, which act similarly to catalysts in that they react with monomers to facilitate polymerisation, but are different as they form part of the polymer as well and are not chemically unchanged at the end. Cracks in the material cause the vessels to crack accordingly, allowing the monomers and agent to flow into the crack site, react together to form a polymer, and repair it.
This method may be more effective as it allows the faster discharge of a greater volume of monomers into the crack site, meaning it can repair larger cracks, and the monomer vessels can repair damage multiple times as monomers flow continuously throughout the network, while the capsules are single-use. However, it is more difficult to implement, and sacrifices the material’s capacity to withstand the strain of heavy loads in the first place, resulting in a weaker material. It is therefore more suited for materials that require continuous use, while capsules are better for disposable products. Below is a visual comparison of the capsule method (top) and the vascular method (bottom):
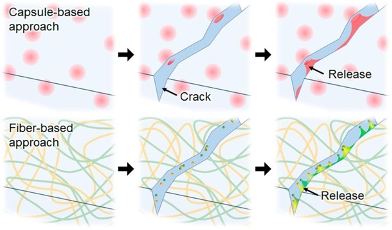
Intrinsic Healing
The final method is known as intrinsic healing. In this case, unlike the other two methods, the ability to self-heal is an inherent chemical property of the material, as opposed to being facilitated by the release of separate stored chemicals into the crack. This may be done by reversible bonds, in which broken bonds in the material’s polymers can reform under the right conditions, usually the same conditions that allowed the polymers to be formed from its monomers in the first place. Furthermore, if the material consists of cross-linked polymers (i.e. parallel linear polymer chains connected by functional groups sticking out of the side), these cross-linking bonds can be reformed as well.
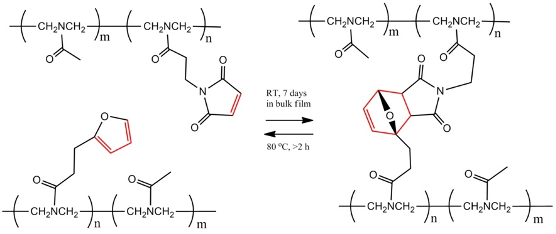
One example of a reversible reaction used in intrinsic self-healing polymers is the Diels-Alder (or DA) reaction, shown below. It involves the formation of covalent bonds between two functional groups known as furan and maleimide (both displayed below in red on the two leftmost molecules, with furan on the bottom chain and maleimide on the top chain), and is used to cross-link polymers in some plastics. When heated to 80°C for two hours in a polar solvent, the cross-linking bonds break, leaving separated polymers. However, if left at room temperature for seven days, those bonds reform and the plastic restores its original properties. This means the plastic can be easily recycled, with the polymers separated, extracted, and reconnected at a processing plant, and the plastic largely retaining its mechanical properties.
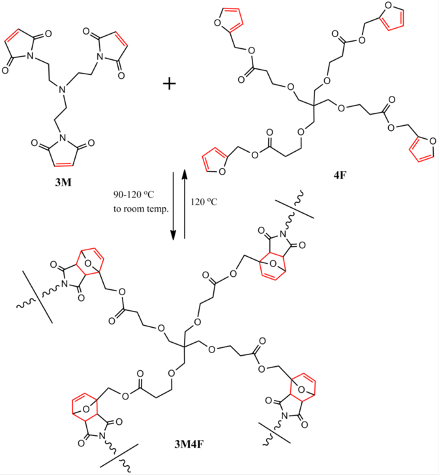
The DA reaction can even form the polymer itself, rather than simply being responsible for the cross-linkage of existing polymer chains. In this case, heating the polymer to 120°C completely depolymerises it via a process known as the retro-Diels-Alder reaction (or RDA), essentially the opposite of the DA reaction, leaving behind the starting monomers. Reheating the monomers to 90-120°C and cooling back to room temperature then repolymerises the monomers, once again via the DA reaction. While the reformed polymer’s mechanical properties are only partially restored, the material can still automatically repair cracks and damage to an extent if the correct conditions are provided. The reaction can be seen here (3M is a compound known as tris-maleimide, and 4F is tetra-furan – the polymer at the bottom involves attaching a 3M molecule to each end of the 4F molecule, allowing the functional groups present to undergo the DA reaction and combine to form the polymer):
In aircraft surface coatings, these DA-based polymers are used as they can heal their own cracks when heated. When cracks appear, technicians use heat guns to break down the ends of surrounding polymer chains via the RDA reaction, leaving behind monomers that flow into and fill the crack. Then, upon cooling back down to room temperature, the DA reaction connects the monomers to the surrounding polymers and each other, filling the hole by reforming the original continuous polymers, so it seems as if no crack had ever existed.
The DA reaction is not the only polymerisation reaction that can be used in intrinsic self-healing polymers. Many others, such as disulfide bonding, share the same reversible property. In fact, a material known as poly(urea-urethane) has even been shown to be able to meld itself back together at room temperature after being cut in half, without the addition of any other chemicals. However, its low tensile strength means it is difficult to use commercially, and increasing tensile strength often means sacrificing the effectiveness of the self-repair mechanism.
This intrinsic self-healing method is convenient as it avoids the need to purchase separate healing chemicals, and the repair can be redone multiple times. The light weight and potential thinness of the materials, in contrast to the bulkiness required for the capsule or vessel self-healing methods, also makes it particularly useful for coatings in electronic or aerospace engineering. However, as we have seen, despite the fact that no separate catalyst or healing agent is needed, these materials often require specific conditions, like temperature, to repair damage to themselves. As such, despite still being revolutionary, the intrinsic self-repair is not truly autonomous, and unlike the external self-healing methods, some types still require a level of human intervention for repair.
Here is a final comparison of the three methods:
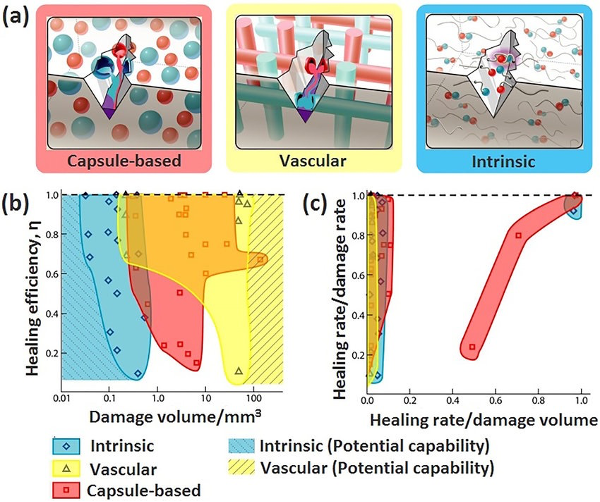
Despite the science-fiction impressions most people get when they imagine self-healing materials, they are, in fact, widely used today, in fields ranging from construction and electronics to transport and medicine. With researchers constantly on the race to optimisation and projections that the global market for self-healing materials will reach $4.1 billion by 2027 with an annual growth rate of 25%, it is undeniable that not only have these materials been a breakthrough in the boundary between science and fiction since 2001, but their usage is likely to only propagate further in the coming years. The next time you throw your video game controller, remember that one day, these materials may save you the £50 required to buy your next.