Rubber Band Fridges
Imagine cooling your food with nothing more than a rubber band. It sounds like science fiction, but the elastocaloric effect can potentially revolutionise refrigeration.

Introduction
The environmental impact of the chlorofluorocarbons (CFCs) and hydrofluorocarbons (HFCs) used in standard refrigerant liquid cannot be understated. CFCs are up to 10,000 times more potent than CO\(_2\) as a greenhouse gas, resulting in refrigerants being currently responsible for 7.8% of global warming. They are so potent due to their ability to absorb and re-emit wavelengths of light on the infrared spectrum which CO\(_2\) cannot. Although there are more environmentally friendly refrigerant alternatives such as hydrofluoroolefins (HFOs), they are much more costly to produce. A cheaper method of refrigeration, with the possibility of a higher coefficient of performance in cooling, is electrocaloric refrigeration. As opposed to exploiting the latent heat of evaporation or condensation of refrigerant fluid, electrocaloric refrigeration leverages the niche property of elastocaloric materials such as rubber or shape memory alloys (SMAs).
The elastocaloric effect can be observed in everyday items like the rubber band: if you stretch a rubber band, contract it again and quickly hold it to your lips, it will feel colder than it was originally. Some people have used this effect to create rubber band fridges, although these are not so much for practical use, but instead to illustrate the concept:
How does the Elastocaloric effect work?
Research has shown that the SMA NiTi, or nitinol, is the most promising elastocaloric material with applications for refrigeration. Nitinol is an alloy made of 55% nickel and 45% titanium. It can achieve a temperature change of up to 15K in either direction of its original temperature through phase transformation. It is also a suitable material as it has a long fatigue life.
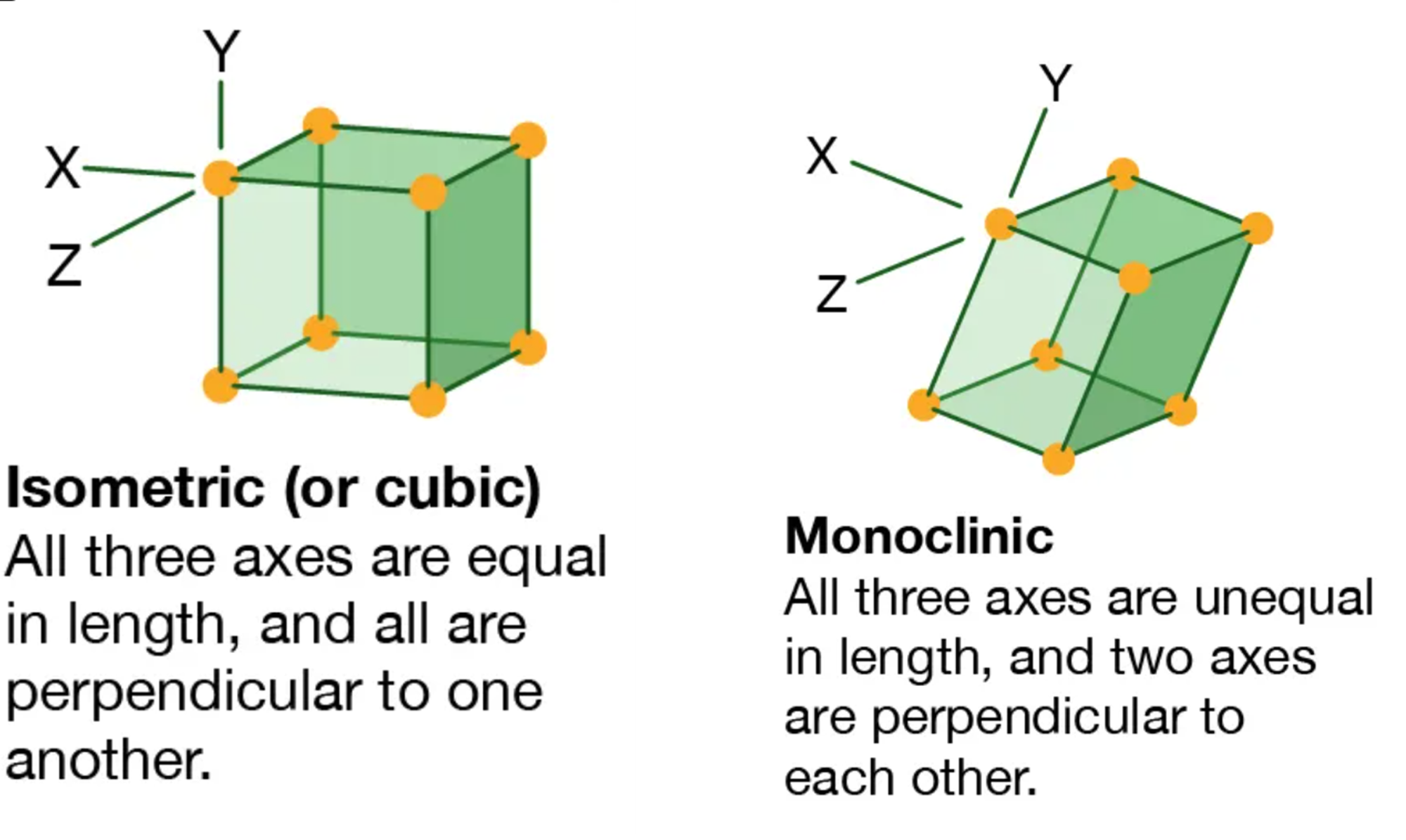
Elastocaloric materials work by having two possible phases which they can adopt in their solid state : martensite and austenite. The martensite phase is triggered by high stress conditions. In the martensite phase, the material is more malleable and has a less ordered B19' monoclinic structure. It also releases latent heat. However, the austenite phase is triggered by low stress. In the austenite phase, the material becomes highly ordered with a B2, high symmetry, cubic structure. The diagrams below illustrate the cubic and monoclinic crystal structure types:
When NiTi is stretched and has a phase transformation from austenite to martensite, it warms up. A phase transition in the other direction causes the NiTi to cool down. Why is this? In short, austenite has more internal energy than martensite. Internal energy is defined as the total energy stored in a system (kinetic and potential). Kinetic energy is the vibration of atoms, while the potential energy is stored in the bonds and relative distances between them. Therefore, when austenite transforms into martensite, its internal energy decreases. However, due to the first law of thermodynamics, energy cannot be created or destroyed. Thus, it must release some energy to compensate for the decrease in internal energy. The opposite applies to the phase transformation from martensite to austenite.
You might wonder why austenite has higher internal energy than martensite. To understand this, you must understand more about the structures of the two phases. When austenite is put under stress, it undergoes diffusionless phase transformation. This means that the individual atoms do not move great distances during the transformation. Because of this, martensite has a great deal of bond strain and dislocations; during the transformation, the atoms do not have the mobility to plug the gaps. Therefore, martensite is less densely packed and less ordered than austenite.
The reason why this is significant is thermal stress. When a material gains or loses heat, it feels a force to expand or contract accordingly. In a disordered material with many dislocations such as martensite, the energy from thermal stress goes into the plastic deformation. However, due to the ordered structure of austenite, plastic deformation does not occur as all the bonds are of equal length and, therefore, strength. Because there are no points of relative weakness, the bonds between the atoms instead stretch and store elastic potential energy, increasing the internal energy of the system.
The Design of a Practical Elastocaloric Refrigerator
The Department of Mechanical and Aerospace Engineering of the Hong Kong university of science and technology have designed an air cooler which has a COP of 3.7, which is comparable to the COP of most air conditioners (3.5). Contrary to expectations, it doesn't work by stretching nitinol linearly, but rather by coil bending as seen in the diagram below:
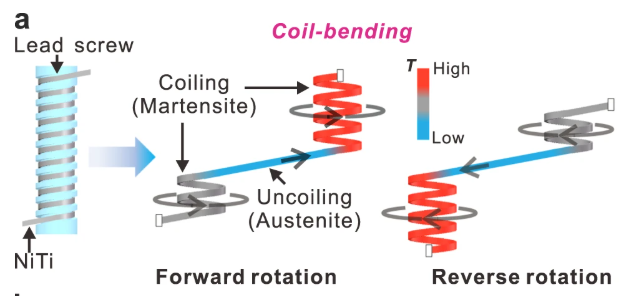
Coiling achieves the same result of phase transforming the austenite in martensite, but in a much more compact volume. Furthermore, by coiling the material around a lead screw, which is thermally conductive, the heat generated can be quickly carried away, generating a steeper temperature gradient.
To extend cooling beyond the 15K temperature change of the individual nitinol wires, several are put into series to amplify the cooling effect as shown below:
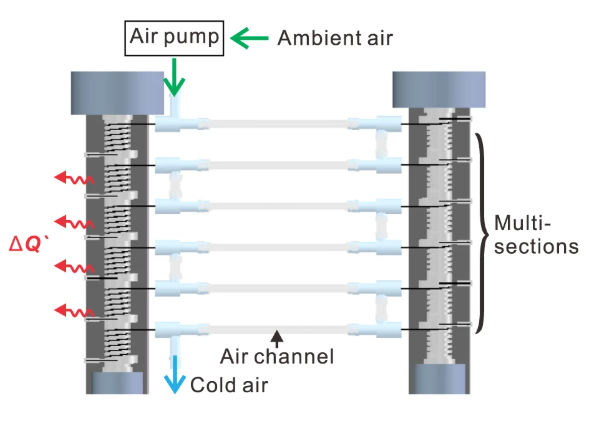
To reduce hysteresis (which is the loss of energy when the elastic potential energy stored in the material is lower than the work put in), copper is added to the alloy in small amounts. The addition of copper reduces the mismatch between the parent phase and the child phase which reduces the amount of strain the material has to go through to change phase. Less strain means less force required which means less work that needs to be done to achieve the same result. Because of the addition of copper and the reduction of strain, the material also increases its fatigue limit (the number of cycles before fracture) to \( 2\times 10^7 \) cycles.
Despite the advancement of the technology, there are reasons for why elastocaloric refrigeration have not replaced standard vapour compression technology. Firstly, nitinol only has a lifespan of about 20 million cycles which lasts just over 2 years if the machine is run at around 0.28 Hz. This is dwarfed by vapour compression refrigerators which can run for over a decade. Furthremore, nickel and titanium are relatively expensive. Until a cheaper material with a longer fatigue life is developed, elastocaloric refrigeration will not be adopted commercially. A potential area to exploe is the opportunity to increase the COP of the technology by recapturing stored elastic potential energy in the coiled nitinol during unloading by using electric generators. However, only time will tell whether the technology will eventually make the leap from a niche gimmick to an industry standard.
Further Reading:
Author links open overlay panelC.H. Fu a, et al. “Austenite–Martensite Phase Transformation of Biomedical Nitinol by Ball Burnishing.” Journal of Materials Processing Technology, Elsevier, 28 July 2014, www.sciencedirect.com/science/article/abs/pii/S0924013614002787.
Author links open overlay panelR. Bennacer a b, et al. “Refrigeration Performance and the Elastocaloric Effect in Natural and Synthetic Rubbers.” Applied Thermal Engineering, Pergamon, 11 Dec. 2021, www.sciencedirect.com/science/article/abs/pii/S1359431121013594.
“Chlorofluorocarbon.” Wikipedia, Wikimedia Foundation, 25 Jan. 2025, en.wikipedia.org/wiki/Chlorofluorocarbon.
Connor, Nick. “What Is Coefficient of Performance - Cop - Refrigerator, Air Conditioner - Definition.” Thermal Engineering, 3 June 2019, www.thermal-engineering.org/what-is-coefficient-of-performance-cop-refrigerator-air-conditioner-definition/.
Li, Xueshi, et al. “Continuous and Efficient Elastocaloric Air Cooling by Coil-Bending.” Nature News, Nature Publishing Group, 2 Dec. 2023, www.nature.com/articles/s41467-023-43611-6.
MechStudies. “What Is Refrigeration Cycle? Basic, Components, Diagram & Explained in HVAC.” Www.Mechstudies.Com, 11 Dec. 2023, www.mechstudies.com/what-is-refrigeration-cycle-basic-parts/.
“Monoclinic System.” Encyclopædia Britannica, Encyclopædia Britannica, inc., www.britannica.com/science/monoclinic-system. Accessed 28 Jan. 2025.
Sewak, R., and C. C. Dey. “Martensitic Phase Transformation in Tini.” Nature News, Nature Publishing Group, 18 Sept. 2019, www.nature.com/articles/s41598-019-49605-z.
“Twisted Elastic Fibers Could Cool Your Food, Study Finds.” Slashdot, science.slashdot.org/story/19/10/11/2324225/twisted-elastic-fibers-could-cool-your-food-study-finds. Accessed 28 Jan. 2025.
Wiley Online Library | Scientific Research Articles, Journals, ..., onlinelibrary.wiley.com/doi/10.1002/aenm.201500361/full. Accessed 28 Jan. 2025.