Problem Of the Week: 20th September 2024
A hot air balloon uses a gas flame to heat the air it contains to a temperature required to enable it to hover at a small distance above ground level. The mass of the balloon, ropes, basket, and riders is 240 kg and the volume of the balloon is 1100 m³. The temperature of the surrounding air is 15°C
Legacy articles aren't reviewed and may be incorrectly formatted.
Problem Of the week: 20th September 2024
Rajas N
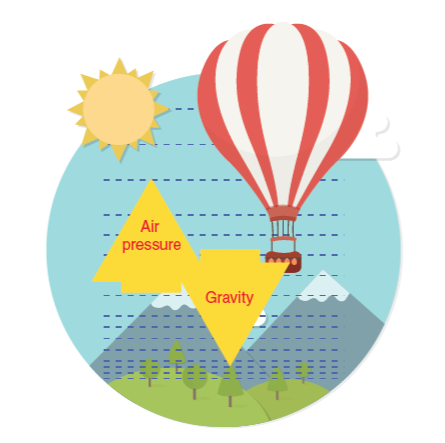
A hot air balloon uses a gas flame to heat the air it contains to a temperature required to enable it to hover at a small distance above ground level. The mass of the balloon, ropes, basket, and riders is 240 kg and the volume of the balloon is 1100 m³. The temperature of the surrounding air is 15°C and its density is 1.23 kg/m³
To what temperature does the air in the balloon need to be heated?
Hint 1 The buoyant force is equal to the weight of the air displaced Hint 2 The relationship between density and temperature is:
Density A / Density B = Temperature B / Temperature A Solution We know the buoyant force is equal to the weight of the air displaced so
M of displaced air = density of surrounding air x V of balloon, so
buoyant force = density of surrounding air x V of balloon x acceleration due to gravity
The total mass of the balloon system is the balloon’s mass + the mass of the surrounding air so
Mass of balloon system = 240 kg + density of inside of balloon x V of balloon
Since it is hovering, the buoyant force is equal to the mass of the balloon system x g
So the whole equation is
(240 + density of inside of balloon x V of balloon) x g = density of surrounding air x V of balloon x g
We can cancel g from both sides so
240 + density of inside of balloon x V of balloon = density of surrounding air x V of balloon
So after rearranging,
density of inside of balloon = density of surrounding air – (240 / V of balloon)
So, density of inside of balloon = 1.23 – (240 / 1100) = 1.012 kg/m³
The relationship between density and temperature is this:
Density of inside of balloon / density of surrounding air =
temperature of surrounding area / temperature of inside of balloon
Hence in kelvin, 15°C = 288°K,
temperature of inside of balloon = (288 x 1.23) / 1.012 = 350°K
350°K = 77°C
Thus, the air in the balloon needs to be heated to 77°C