How Neuroactivating Substances Change The Brain
Rising stimulant use among young adults raises a vital question: what do these drugs really do to the brain? This article uncovers how substances like cocaine, and even caffeine, rewire emotion, motivation, and control, revealing how short-term highs can leave lasting marks on the mind.

As illegal stimulant use among 16–to 24-year-olds in the UK rises to nearly 3.8% in 2024 (ONS, Centre for Crime and Justice), questions grow about what these substances are actually doing to the brain. Young, developing brains are particularly vulnerable to rapid and long-term changes in memory, emotional regulation, and even cognitive capacity. This article explores how Class A stimulants interact with the brain’s systems, particularly the limbic system, which regulates emotion, examining case studies and neuroscience to uncover how short-term highs may shape long-term neurobiology. To complement this, I will conduct a primary investigation into the effects and withdrawal experience of a legal stimulant: caffeine. Despite their differences in legality and strength, all stimulants share the common effect of excessively activating the brain’s emotional and motivational circuits, often causing lasting consequences.
Section One- Primary Action
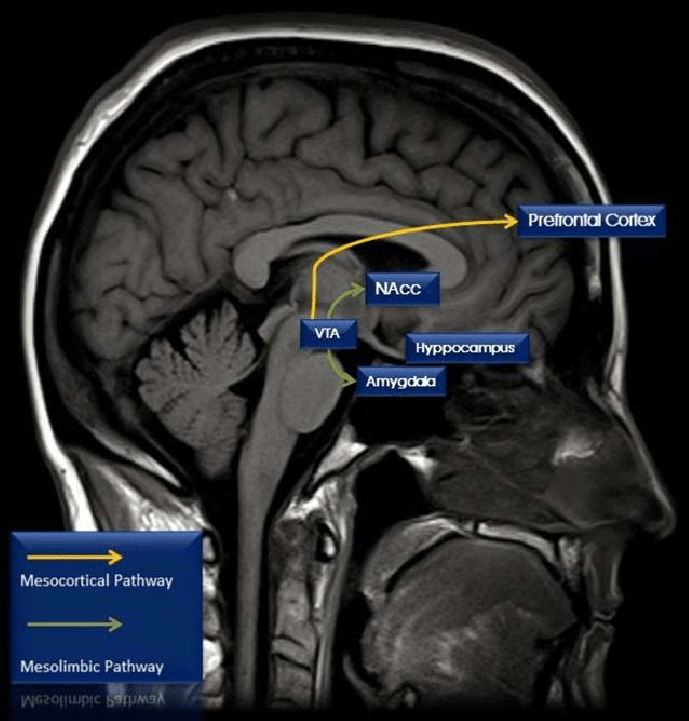
Cocaine is a psychostimulant, meaning it temporarily boosts brain activity. When someone first takes cocaine, they often briefly feel happier, more confident, alert, and physically energised. Cocaine affects several neurotransmitters in the brain, but its main impact is on a group called the monoamines, especially dopamine, which is heavily involved in feelings of reward and pleasure. The brain circuit most affected by this is called the mesocorticolimbic system, seen in Figure 1, which normally activates in response to everyday pleasurable experiences. This pathway starts in the ventral tegmental area (VTA), which is a part of the midbrain and contains neurons responsible for the release of dopamine in order to interact with areas like the nucleus accumbens and the medial prefrontal cortex, both of which are important for motivation, decision-making, and emotion. Cocaine and similar drugs disrupt this system by stopping the reabsorption of dopamine through blocking dopamine transporters. This causes a buildup of dopamine, which strongly boosts the brain's reward signals, and causes the previously mentioned effects of alertness and happiness.
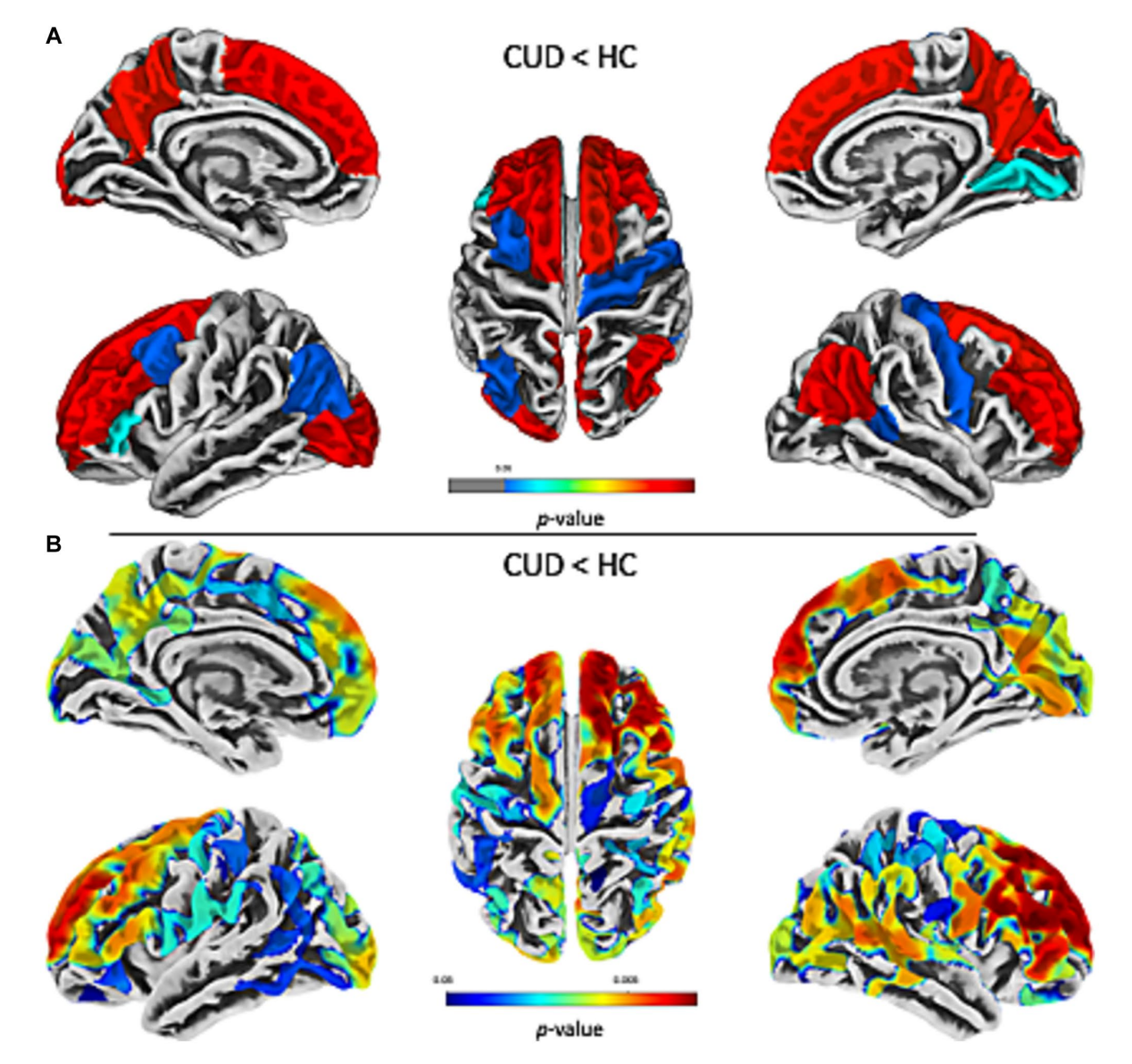
Over time, repeated cocaine use not only disrupts chemical signalling in the brain but also alters its structure. Normally, the brain maintains a balance between neurons that encourage reward-seeking and neurons that inhibit impulsive actions. In addiction, the inhibitory neurons lose effectiveness, while the reward-driven neurons become hypersensitive, intensifying cravings and compulsive drug-seeking. MRI studies, as seen in Figure 2, show that chronic cocaine users also develop thinning in the cortex, particularly in areas such as the prefrontal and temporoparietal regions, which are critical for decision-making, emotional regulation, and self-control. These structural changes reinforce the functional imbalance: the circuits that usually help regulate motivation and behavior are physically compromised, making it harder to resist cravings.
Furthermore, according to this study, chronic use of cocaine can even accelerate “brain ageing”, with users’ brains appearing biologically older than their chronological age, demonstrating how overstimulation of reward pathways has long-term consequences on both brain structure and function. (Schinz, D., et al. (2023))
Section Two- Chemical Comparison
In this section, I will compare the chemical activity of cocaine to that of the common neuroactivating substance: caffeine. At the chemical level, both caffeine and cocaine act through interactions with receptors and transporters, altering the normal flow of neurotransmitters across synapses.
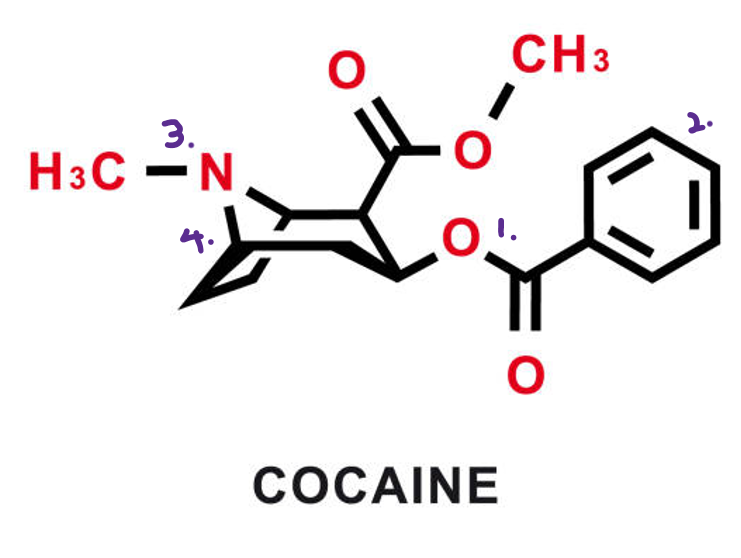
Cocaine belongs to the ‘tropane alkaloid’ family, typically containing ester (1), cyclo/benzene (2), and tertiary amine (3) groups with a bicyclic structure (4) that allows it to bind directly to the dopamine transporter, preventing dopamine from re-entering the presynaptic neuron. The blockade leaves dopamine molecules inside the synaptic cleft, where they continue to stimulate receptors, producing the intense reward and euphoria associated with cocaine’s high. Cocaine also inhibits the transporters for norepinephrine and serotonin, amplifying its effects. (Volkow, N.D., et al. (1997))
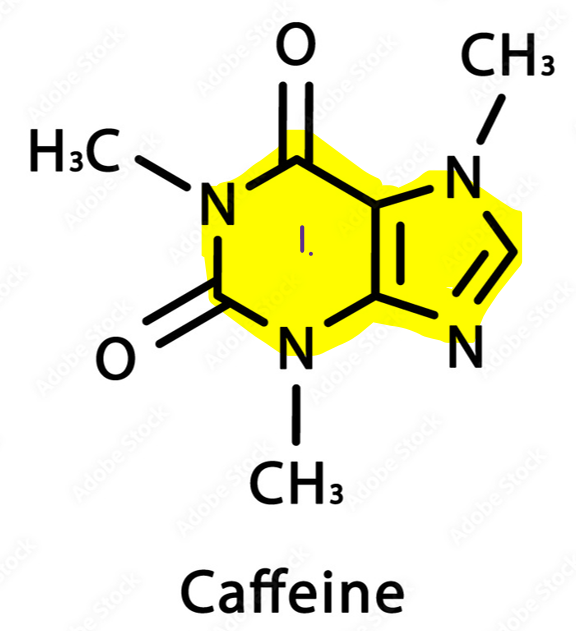
Caffeine, by contrast, is part of the ‘methylxanthine’ family, which is structurally similar to adenosine (the chemical responsible for tiredness). Its purine ring system (1) allows it to bind competitively to adenosine receptors without activating them, effectively functioning as an adenosine antagonist. (Schwarzschild., et al. (2002)) Under normal circumstances, adenosine builds up in the brain during prolonged wakefulness, binding to receptors to promote drowsiness and reduce neural firing. By blocking adenosine, caffeine prevents this “fatigue signal” from occurring, indirectly leading to increased activity of dopamine and other excitatory neurotransmitters. Unlike cocaine, caffeine does not flood the synapse with dopamine, but rather modulates the system indirectly, which explains its milder stimulation and lower addictive potential. (Do, H.N., et al. (2021))
These mechanisms highlight the subtle but crucial differences in how molecular structure determines function. Cocaine’s ability to directly inhibit transporters produces an immediate and overwhelming dopamine surge, making it highly reinforcing and addictive. Caffeine’s action, while still psychoactive, is more regulatory, gently shifting the balance of neural activity without overwhelming the brain’s homeostatic mechanisms.
Section Three- Primary Investigation: Caffeine
To complement the discussion of cocaine, I conducted a self-experiment examining the short-term effects and withdrawal of caffeine, the most widely consumed psychoactive substance worldwide. For 2 weeks, I consumed approximately 400 mg of caffeine daily, equivalent to 3 cups of coffee or energy drinks, and recorded subjective changes in mood, alertness, and concentration. Following this period, I ceased caffeine intake to observe withdrawal effects.
During the period of regular caffeine consumption, I noted increased alertness, enhanced focus, and improved motivation, particularly during cognitively demanding tasks. These observations are consistent with caffeine’s pharmacological action as an adenosine receptor blocker. By blocking adenosine, a neurotransmitter that promotes sleepiness and relaxation, caffeine reduces the brain’s perception of fatigue and indirectly enhances dopaminergic activity in reward pathways. Cocaine, on the other hand, as mentioned, acts directly on dopamine transporters, preventing reuptake and causing a large and rapid buildup of dopamine in synapses.
Upon cessation of caffeine, withdrawal symptoms emerged within 24 hours. These included headaches, irritability, and difficulty sustaining concentration, continuing for approximately 3 days before gradually resolving. This rapid onset and alleviation of symptoms illustrate the brain’s ability to adapt to repeated stimulant exposure and the potential for dependence, even with substances generally regarded as safe.
With caffeine, 400 mg per day was enough to produce noticeable stimulation and withdrawal within days, but the symptoms were temporary and resolved the brain readjust. With cocaine, even small doses can override the brain’s natural control systems and create rapid dependence which is difficult to overcome without serious withdrawal symptoms of headaches, insomnia and depression.
By comparing caffeine and cocaine I want to highlight how stimulants, whether legal or illicit, can modify brain chemistry, influence behavior, and if misused, exert lasting effects on motivation, cognition, and emotional regulation. Therefore, I urge the reader to truly think about what they are subjecting their brain to, and to always keep everything in moderation, whether sugar, caffeine, alcohol, tobacco or any other drug to prevent dependencies and damage, which may prove difficult to reverse.
Bibliography
Alamy Limited (2024). Cocaine chemical formula. Cocaine chemical molecular structure. Vector illustration. [online] Available at: https://www.alamy.com/cocaine-chemical-formula-cocaine-chemical-molecular-structure-vector-illustration-image456525729.html [Accessed 28 September 2025].
Mavink.com (2024). Mesocortical pathway. [online] Available at: https://mavink.com/explore/Mesocortical-Pathway [Accessed 10 July 2025].
Office for National Statistics (ONS), Centre for Crime and Justice (2024). Drug misuse in England and Wales: year ending June 2024. [online] Available at: https://www.ons.gov.uk/ [Accessed 28 September 2025].
Schinz, D., Koenig, J., Zimmermann, R., Hulka, L.M., Preller, K.H. and Herdener, M. (2023). Lower cortical thickness and increased brain aging in adults with cocaine use disorder. Frontiers in Psychiatry, 14, 1266770. doi:10.3389/fpsyt.2023.1266770. [Accessed 27 September 2025].
Shatilova, I. (2020). Caffeine structural chemical formula on white background. [online] Dreamstime. Available at: https://www.dreamstime.com/caffeine-structural-chemical-formula-white-background-caffeine-structural-chemical-formula-white-background-image195365456 [Accessed 28 September 2025].
Volkow, N.D., Wang, G.-J. ., Fischman, M.W., Foltin, R.W., Fowler, J.S., Abumrad, N.N., Vitkun, S., Logan, J., Gatley, S.J., Pappas, N., Hitzemann, R. and Shea, C.E. (1997). Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature, 386(6627), doi:https://doi.org/10.1038/386827a0. [Accessed 26 September 2025].
Schwarzschild, M.A., Chen, J.-F. and Ascherio, A. (2002). Caffeinated clues and the promise of adenosine A2A antagonists in PD. Neurology, [online] 58(8), pp.1154–1160. doi:https://doi.org/10.1212/WNL.58.8.1154. [Accessed 24 September 2025].
Do, H.N., Akhter, S. and Miao, Y. (2021). Pathways and Mechanism of Caffeine Binding to Human Adenosine A2A Receptor. Frontiers in Molecular Biosciences, 8. doi:https://doi.org/10.3389/fmolb.2021.673170. [Accessed 28 September 2025].
Schinz, D., Schmitz-Koep, B., Tahedl, M., Timo Teckenberg, Schultz, V., Schulz, J., Zimmer, C., Sorg, C., Gaser, C. and Hedderich, D.M. (2023). Lower cortical thickness and increased brain aging in adults with cocaine use disorder. Frontiers in Psychiatry, 14. doi:https://doi.org/10.3389/fpsyt.2023.1266770. [Accessed 28 September 2025].