Alfred Nobel's Life and Legacy
An introduction to the complicated life and contrasting legacy of the man behind the prizes.

Introduction
Picking up a French newspaper in 1888, Alfred Nobel was surprised to see his own obituary within its pages, evidently written after the publisher had mistaken Ludvig Nobel's death for his younger brother's. Titled “le marchant de la mort est mort” (the merchant of death is dead), the scathing article stated that "Dr. Alfred Nobel, who became rich by finding ways to kill more people faster than ever before, died yesterday". How had the Swedish inventor built such a sombre reputation and what could he do to amend it in the years he had left?
Early Life and Education
Born on the 21st October 1833, Alfred was the third of Immanuel Nobel’s four surviving sons. Having been forced into bankruptcy by financial struggles in his entrepreneurial business endeavours mere months before Alfred’s birth and with creditors hot on his heels, Immanuel moved from Sweden to St Petersburg in 1837, where the rest of the Nobel family would join him 5 years later. Immanuel saw much more success as an inventor and industrialist in Russia over the coming decades, most notably from the invention of the rotary lathe – a carpentry devise used in the production of plywood - and from the manufacture of naval mines and armaments for Tsar Nicholas 1st 's government. This newfound economic prosperity meant that the family could pay to have Alfred educated by a number of private tutors, including the Russian chemist Nikolay Zinin.
Being a sickly child from birth, Alfred spent the majority of his childhood indoors with a book and was always considered the most academically promising of his sons by Immanuel, who encouraged him to pursue a scientific career instead of focusing on the study of poetry and literature, in which he would continue to have a keen interest throughout his life. By his teenage years Alfred began to show a particular affinity for chemistry and languages, reportedly achieving fluency in Swedish, English, French, Russian and German. Under Zinin’s recommendations, Alfred was sent to Paris in 1850 for further chemistry studies in Jules Pélouze’s lab; it was here that he would meet Ascanio Sobrero - the discoverer of a novel explosive compound which would come to be the centrepiece of Alfred’s life’s work: Nitroglycerin.
Nitroglycerin
Working at the University of Turin in 1846, Ascanio Sobrero discovered that reacting glycerol with concentrated nitric and sulphuric acid produced a translucent oily liquid with remarkable explosive qualities and incredible shock sensitivity, calling the new chemical “pyroglycerin” after the Greek word for fire. The Italian chemist argued vehemently against the use of pyroglycerin, believing it to be “extremely dangerous and impossible to handle safely”; so terrified was he by the destructive potential of his discovery that he initially attempted to keep it concealed from the scientific community. In later life Sobrero would say “When I think of all the victims killed during nitroglycerin explosions, and the terrible havoc that has been wreaked, which in all probability will continue to occur in the future, I am almost ashamed to admit to be its discoverer.” Clearly, the Nobel family did not share his cautiousness.
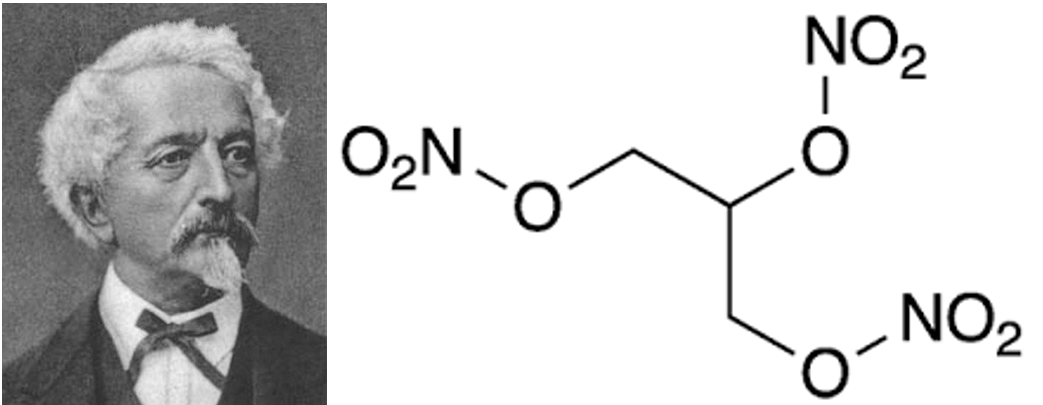
Nitroglycerin (trinitroglycerol) was a new type of high explosive that was many times more powerful than black powder – a mixture of phosphorous, saltpetre and charcoal which had been the most potent useable explosive for centuries. The key difference between a high explosive like nitroglycerin and a low explosive like black powder is the speed at which the reaction propagates through the material: whereas black powder deflagrates when heat is applied, with combustion moving through the material at subsonic speeds, nitroglycerin detonates - the explosion spreads through the material at near-instant speeds of just under 8000m/s. Expectedly, such a high detonation velocity results in the formation incredibly powerful pressure waves with an excellent capability for shattering and pulverising. It is worth mentioning that nitroglycerin is also used in pharmaceutics as a vasodilator; in an ironic twist of fate, Nobel himself was administered medicinal nitroglycerin later on in his life.
The presence of Nitrogen, Oxygen, Carbon and Hydrogen within the molecule allows nitroglycerin to “act as its own oxidiser”, thus allowing the explosion to spread through the substance without needing to wait for exposure to the oxygen in the air. When looking at the chemical equation for the decomposition of nitroglycerin: 4C3H5N3O9 → 12CO2 + 10H2O + 6N2 + O2 , we can see that the reaction of 4 moles of the unstable molecule produces 29 moles of smaller gaseous molecules, resulting in a rapid expansion of hot gases during the detonation. Moreover, the products of the reaction are all stable molecules with high bond enthalpies, which makes this reaction highly exothermic, releasing 1414kJ/mol or 6.23kJ/g - an energy density 50% greater than that of TNT (usually the benchmark for assessing explosives).
Dynamite
Enamoured with the new explosive, Alfred was eager to find a way of “taming” nitroglycerin and turning into a commercially viable explosive for use in mining and industry. Returning to St Petersburg in 1852, Alfred told his father about his new passion, on which the two would work during their time away from running the armaments factory. The following years were somewhat of a golden age for the Nobel family, with their business flourishing as a result of the increased demand for armaments brought about by the Crimean War; Immanuel’s naval mines being put to good use in the defence of Kronstadt’s naval base and the inventor himself receiving the Imperial Gold Medal for his contribution to Russian industry.

With the end of the war came a reduction in military spending by Tsar Alexander 2nd and a downturn in the Nobels’s finances, who struggled to shift their factory to the production of civilian goods; in 1859 Immanuel left what remained of the St Petersburg business in the care of his son Ludvig and moved to Stockholm with his Alfred and his mother, where they would be able to solely focus on the production and exploitation of nitroglycerin.
The first hurdle that needed to be overcome was finding a way to safely detonate the nitroglyerin from a distance; unlike gunpowder, which can be set off by the heat of a flaming fuse, detonating explosives like nitroglycerin usually require a primary shock to initiate the explosion. Nobel’s soltion - the invention of the blasting cap - was one of the most significant breakthroughs in the history of explosives and arguably one of his two greatest scientific contributions. The first detonator he came up with in 1863 consisted of a wooden cylinder containing a charge of gunpowder which could be inserted into a larger vat of nitroglycerin and ignited with a fuse to trigger the greater secondary explosion. He would go on to improve this design in 1865, settling on a copper blasting cap containing a small charge of mercury fulminate – another high explosive which is famously used by Walter White in the 2nd Season of “Breaking Bad”. Variations of Nobel’s blasting cap would continue to see widespread use in civilian and military spheres for decades to come.

The second problem with taming nitroglycerin became all-too-evident on 3rd September 1864 when batch of nitroglycerin exploded at the Nobels’s Heleneborg factory in Stockholm, tragically resulting in the deaths of five people, including Emil Nobel, Alfred’s younger brother. Clearly, the instability of pure liquid nitroglycerin made it impossible to be safely transported and handled without risking future catastrophes, as indeed happened in April 1966 when a crate of nitroglycerin detonated in a San Francisco warehouse. However, rather than dissuading Alfred, the death of his brother only served to spur him on to redouble his efforts in making nitroglycerin safer to use, opening several new factories across Germany and Sweden and even working on a barge moor in Lake Malaren after the Swedish government banned further experimentation within the Stockholm city limits.

Alfred stumbled upon the solution to the stability conundrum while walking on the banks of the Elbe river outside his Krümmel factory in 1866; he had already tried reducing nitroglycerin’s shock sensitivity by combining it with other unreactive substances like sawdust and charcoal but he found diatomaceous earth (the fossilised remains of microalgae) to be the most effect stabiliser. On a microscopic level, a mixture of nitroglycerin with diatomaceous earth was much less sensitive than pure nitroglycerin because the unreactive stabilizer particles, largely consisting of Silica, surrounding a molecule of nitroglycerin could absorb and dissipate the energy released from its breakdown, thus suppressing the spread of an accidental detonation through the material in the form of a chain reaction. Furthermore, the mixture turned out to be a soft dough-like substance which could be easily and safely kneaded into rods for use in mining and construction. Nobel patented a combination of these rods (originally 75% nitroglycerin and 25% stabilizer) with a blasting cap in May 1867, calling it Dynamite after “Dynamos” – the Greek word for power.
Later Life
Naturally, dynamite was beyond commercially successful; being a stable yet powerful explosive, it became irreplaceable in the industries of mining, quarrying, tunnelling and construction, spreading around the globe and making its creator fabulously wealthy. Nobel set up a vast network of over 90 factories across 20 countries and filed 355 patents to protect the profits from his inventions; by the end of his life he had amassed a fortune equivalent to around $200 million in today’s money. Part of these assets came from his holdings in Branobel, an oil company based in Baku (a city on the banks of the Caspian Sea in today’s Azerbaijan) which had been founded by the two Nobel brothers who had stayed in Russia after their father’s departure. It is estimated that Branobel could have at one point been responsible for as much as one half the world’s total oil production; it is therefore no surprise that Ludvig Nobel, the major shareholder, became even richer than his younger brother.

Meanwhile, Alfred continued working to improve his product in the ensuing years, perfecting the stabiliser contents and developing two new versions of the explosive. The most important of these came about in Paris when Nobel discovered that collodion-cotton (a fluffy substance mainly composed of nitrocellulose) dissolved in nitroglycerin and mixed with saltpetre and wood pulp produced a hard plastic material that was even more powerful than dynamite and held the added benefit of being waterproof. Patented as Gelignite (blasting gelatin) in 1876, the new explosive was every bit as popular as dynamite and brought just as much money into Nobel’s growing empire. Nobel’s last major invention was Ballistite – a smokeless propellant consisting of camphor, nitroglycerin and nitrocellulose patented in 1887. The new propellant was soon licensed to the Italian military for the outfitting of the cartridges of their M1890 Vetterli rifles; this deal greatly angered the French government, subsequently forcing a move to San Remo in northwest Italy, where Nobel would live out the last years of his life.
Legacy
The extent to which the aforementioned false obituary of 1888 catalysed Nobel’s decision to set up the prizes is still a topic of considerable debate. A self-proclaimed pacifist and a man who loathed the human suffering brought about by war, it is no surprise that Nobel was disturbed by the fact that much of his life’s work had been devoted to the advancement of destructive technology, even if Dynamite itself was generally considered inferior to other explosives like TNT when it came to military applications. One way he tried to reconcile this paradoxical discrepancy in his consciousness was by claiming that his inventions could protect peace by acting as a deterrent against armed conflict: “My dynamite will sooner lead to peace than a thousand world conventions. As soon as men will find that in one instant, whole armies can be utterly destroyed, they surely will abide by golden peace” - a sentiment echoed by the creators of the atomic bomb over half a century later, though one which was proved to be naively optimistic by the Great War two decades later.
Signing his last will and testament in 1895, Nobel bequeathed 94% of his enormous fortune to the setting up of a foundation for the awarding of five annual prizes to the individuals who had “conferred the greatest benefit on mankind”. The first prizes were awarded in 1901, with the five categories chosen being Chemistry, Physics, Medicine, Literature and Peace; a prize for economics was later added in 1969. The Swedish inventor died childless on 10th December 1896 in his San Remo villa; he never married. To this day Nobel’s legacy remains a complex one, telling of a man who’s name will be inextricably associated with the most prestigious prize for the furthering of the peace and progress of humanity, the recipients of which are among the greatest scientific and political minds of the 20th century; yet also a man who, despite his pacifistic ideals, devoted his life to the sale and manufacture of the age’s most deadly explosives.

References
https://www.britannica.com/biography/Alfred-Nobel
https://www.nobelprize.org/alfred-nobel/alfred-nobels-life-and-work/
https://www.mcgill.ca/oss/article/history/how-dynamite-spawned-nobel-prizes
http://cprr.org/Museum/Newspapers/Nitroglycerine.html
https://ambadar.com/insights/patent/alfred-nobel-the-symbol-of-inventiveness/
https://www.nobelprize.org/alfred-nobel/nitroglycerine-and-dynamite/
https://www.britannica.com/technology/explosive/Other-explosives#ref82380
https://www.nobelprize.org/alfred-nobel/immanuel-nobel/
https://www.history.com/news/did-a-premature-obituary-inspire-the-nobel-prize
https://www.interfire.org/res_file/def_det.asp
https://www.ch.ic.ac.uk/rzepa/mim/environmental/html/nitroglyc_text.htm